Eli Lilly’s Oral Weight Loss Pill Orforglipron Shows Strong Results, Rivals Injectables Like Ozempic
Articles
Summary

GLP-1 pill: Eli Lilly says its oral orforglipron showing “promising results”
Lilly — which makes the diabetes drug Mounjaro and the weight-loss treatment Zepbound — said orforglipron is "the first oral small molecule glucagon-like peptide-1 (GLP-1) receptor agonist, taken without food and water restrictions, to successfully complete a Phase 3 trial."
Left
Axios
Weight loss pill just as effective as popular injectables in late-stage trial
Eli Lilly said its weight loss pill Orforglipron has proven just as successful as popular injections in a late-stage trial among type 2 diabetics. On average, trial participants lost 8% of their body weight. The pill was also found to lower blood sugar. Unlike weight loss injections, the oral pill must be taken daily, instead of weekly.
Middle
Straight Arrow News
Eli Lilly stock surges 15% after clinical trial of weight loss pill shows it works like Ozempic
Eli Lilly shares jumped 15% after a clinical trial of its experimental pill showed it helped patients with Type 2 diabetes lose weight at levels comparable to leading injectable medications like Ozempic. The mid-stage trial results announced on Thursday by the pharmaceutical giant showed that patients taking orforglipron lost an average of 16 pounds — or 7.9% of their body weight — over a 26-week period.
Right
New York Post
Daily weight-loss pill could be the new Ozempic
People taking the medication orforglipron lost an average of 16lbs in nine months. Participants, all of whom were obese and had Type 2 diabetes, also saw significant falls in their blood sugar. Phase III trial paves the way for manufacturers to seek a licence for the drug.
Right
The Telegraph
Diabetes and weight-loss drug ‘changed my life,’ says senator: ‘I feel a decade younger’
Sen. John Fetterman, D-Pa., wrote about his experience with Mounjaro (tirzepatide) The injectable prescription medicine is primarily used to treat type 2 diabetes. "I feel a decade younger, as well as clearer-headed and more optimistic than I’d been in years," he said.
Right
Fox NewsHEALTH
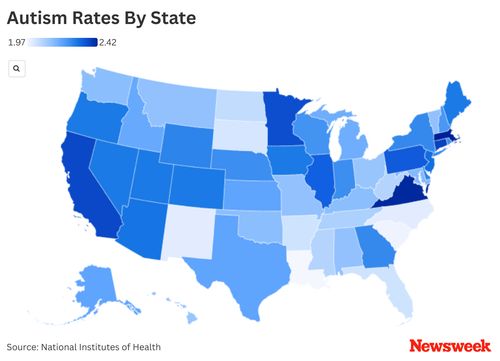
RFK Jr. Disputes CDC Study Claiming Autism Rates Have Risen Due to Improved Screening
Updated: 14 hours ago
Articles
Summary
Articles
Summary
HEALTH
FDA Issues Urgent Warning Over Counterfeit Ozempic for Diabetes and Weight Loss
Updated: 2 days ago
Articles
Summary
HEALTH
North Carolina Faces Deadliest Flu Season on Record With Over 500 Deaths in 2024–2025
Updated: 4 days ago
Articles
Summary