Annual Shot to Protect Against HIV Passes Key Clinical Trial
Articles
Summary

Once-yearly jab for HIV protection passes first trial hurdle
An annual injection designed to guard against Human Immunodeficiency Virus (HIV) has completed an important early safety trial, researchers report in The Lancet medical journal. Lenacapavir stops the virus from replicating inside cells. If future trials go well - now it has passed the first, Phase I, testing hurdle - it could become the longest-acting form of HIV prevention available.
Left
BBC News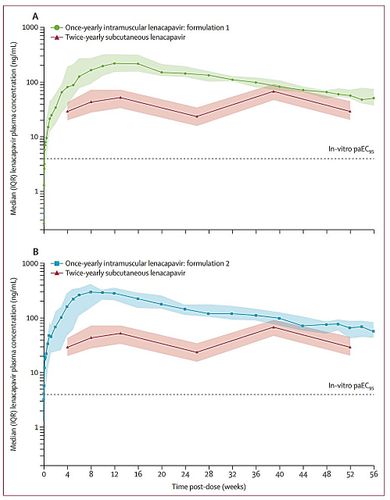
Reformulation of HIV prevention drug lenacapavir makes it last for a year
A team of pharmaceutical researchers at biopharma company Gilead Sciences has announced that a reformulation of its HIV prevention drug lenacapavir allows it to persist in the body for up to a year.
Middle
Medical Xpress
Yearly HIV prevention injection 'Lenacapavir' shows promise in clinical trial
'Lenacapavir' was developed by Gilead Sciences, a research-based biopharmaceutical company in the US. The drug works by blocking HIV from entering and multiplying in human cells. The trial included 40 participants, aged 18-55 years, who did not have HIV.
Middle
The New Indian Express
Gilead Sciences (NasdaqGS:GILD) Advances HIV Treatment With Promising Lenacapavir And Biktarvy Data
Gilead Sciences (NasdaqGS:GILD) recently experienced a significant price movement, rising 24% in the last quarter. This upward trajectory coincides with positive developments in its HIV treatment portfolio, highlighted by successful data presentations at CROI 2025. The approval of lenacapavir for multi-drug resistant HIV and its promising trial results for once-yearly PrEP injections in significant publications emphasized Gilead's innovative edge. Coupled with robust earnings in Q4 2024,...
Middle
Simply Wall StHEALTH
RFK Jr. Calls on Food Company Executives to Remove Artificial Dyes From Their Products
Updated: 3 hours ago
Articles
Summary
HEALTH
New Study Suggests Aspirin Could Be Used to Prevent Certain Cancers From Spreading
Updated: 3 hours ago
Articles
Summary
HEALTH
RFK Jr. Meets with Sean Hannity at Steak 'n Shake to Sample New Beef Tallow Fries
Updated: 16 hours ago
Articles
Summary
HEALTH
Trump Administration Launches $1 Billion Plan to Fight Bird Flu, Lower Egg Prices
Updated: Yesterday
Articles
Summary